Log on to your favorite news site, pick up a newspaper, tune into a nightly news program and most likely you will hear a common refrain: the average consumer's safety is under attack, the businesses serving those consumers don't have the proper safeguards in place, and the regulators are moving in en-masse to fix the problem. The theme is a common one across all segments of society: we don't know what is going on in the banking industry so more controls are needed, the real estate market collapsed due in part to the opaque nature of how mortgages were created and sold and food items like spinach, peanuts, etc. are turning up tainted in the U.S food supply.
The global pharmaceutical industry is far from being isolated from these pressures and sits squarely in the middle of its own growing storm. The blood thinner drug Heparin was recalled due to the presence of a tainted ingredient, an incident that resulted in the deaths of at least 81 people and the sickening of hundreds more. Counterfeit versions of vaccines to prevent HIV/AIDS, malaria, tuberculosis and the flu are repeatedly intercepted by police and customs agents, threatening not only corporate bottom-lines but also the creation of resistant virus strains. With the number of global pharmaceutical safety incidents growing at a rate of 25% per year, topping 1,700 in 2007 according to the Pharmaceutical Security Institute, it is no wonder that regulatory rumblings in the pharmaceutical industry are getting louder than ever.
So are these issues just more of the same or the beginnings of a sea change in the way of doing business in the global pharmaceutical supply chain? Will waiting for the clouds to clear provide greater insight on what to do or does it create the risk of playing chicken with drug safety and regulatory issues. Against this backdrop, several questions emerge. Should pharmaceutical companies sit back and wait for someone else to blaze the drug traceability trail? Given the history of regulatory delays and modifications, can we expect to see the current regulatory mandates be pushed back again? What should pharmas consider as they plan and execute drug traceability solutions and what technologies should they use?
Drug Traceability Mandates: The Global Drumbeat Continues
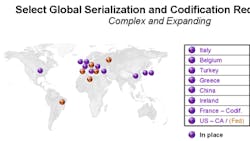
A quick survey of the global regulatory landscape hints at the scope of the challenges facing multinational pharmaceutical companies, the supply partners that serve them and the trading partners that buy from them. Some of the challenges include:
- Monthly occurrences of counterfeit and diverted drug seizures.
- Continued incidences of healthcare reimbursement fraud and revenue leakage.
- Infrequent but potentially devastating episodes of product recalls and market withdrawals that highlight the opaque nature of the drug supply chain.
- Increasing drug costs and demands for healthcare expenditure control.
These have all led to a patchwork of serialized product identification, global traceability and new codification requirements for pharmaceutical companies and other industry participants to contend with.
In an attempt to address these challenges, numerous countries in the European Union currently have on the books or are proposing product traceability and reporting requirements by the middle of the next decade, driven by a diverse mix of goals. Turkey has put into place item-level serialization requirements married to a set of ten different governmental notification reports while companies targeting the markets of Italy and Belgium face a different set of reporting requirements for serial numbers put into the market and quantities sold. France's CIP-13 codification efforts which incorporate expiration date and batch number, when completed, will bring yet another large swath of the EU market demand under the drug traceability umbrella. Not to be left behind, Brazil, Columbia, Serbia and Spain appear poised to institute pharmaceutical serialization programs in the near future. These regional operational requirements will only get stiffer as governments and industry participants begin to respond to the recently announced European Commission proposals for increasing the transparency of the drug supply, the ingredients that feed into it and the safety of the products produced by it.
Turn the Heat Up: The U.S. Moves to Increase Security
In contrast to other regions, the focus on drug traceability in the United States has been fairly narrow until very recently. Starting with the original Pharmaceutical Drug Marketing Act (PDMA) of 1987 and continuing into this decade with two dozen or so state-level programs, the thrust of domestic regulations was centered on establishing documentation of a secure chain of custody, also known as a drug pedigree. California decided to go one step further and include an item-level drug serialization requirement in their state mandates. Given the significant incremental investment this would require, it was no surprise when the start date for CA compliance was pushed back to 2015, giving the industry a sigh of relief. But should it? A closer look at the current domestic landscape should give pause to anyone feeling too comfortable.
Several recent federal actions have been taken that lay the foundation for broadly increased traceability requirements. The FDA recently published draft guidance for a Serialized Numerical Identifier (SNI) standard for drug packages. The SNI standard, finalization, which is required by March 2010 according to the FDA Amendment Act of 2007 (FDAAA), will help address one element of the finished dose serialization question. The FDA and the Department of Homeland Security jointly published draft guidance on Good Importer Practices to ensure that imported products are in compliance with applicable U.S. statues and regulations, including drugs, ingredients and components that are foreign-sourced as well as US-manufacturer products that are re-imported into the U.S. In addition, FDA in cooperation with US Customs and Border Protection (CBP) outlined a voluntary Drug Import Secure Supply Chain (SSC) Pilot Program in January 2009. The goals are to determine the practicality of expediting imports of finished drugs and APIs while assisting FDA's efforts to prevent the importation of adulterated, misbranded and unapproved drugs by enabling FDA to focus resources on imported drugs that may pose higher risks. Finally, several initiatives are percolating around in the U.S. Congress, including the "FDA Globalization Act of 2009" that targets increased FDA authority to improve the safety of imported drugs, devices, food and cosmetics.
Why Act Now?
Until now, against this uncertain backdrop, the general reaction has been to take one of two approaches. First, companies with no immediate compliance need willwait as long as possible in expectation that general harmonization of technology, process and regulatory requirements will occur on their own, all while hoping that the deadlines will be extending again. Second, companies with immediate compliance needs will take a tactical, stop-gap approach focused on meeting the compliance bar for each new deadline by minimizing the business units and products impacted. Under this approach, outsourced partners are often enlisted to "slap and ship" required labels and manage local reporting requirements. Both approaches reduce cost in the short run but leaves us wondering if this is a sustainable model or is now the time to embark on development of a strategic, phased plan that encompasses known needs and leaves flexibility for the unknown?
Having a strategic plan to meet global traceability and serialization requirements can result in multiple benefits including the following:
- Operational Improvement
Firms have an opportunity to develop, exercise and share best practices across business units and regions. Questions exist across a broad range of technology, process and integration issues. A continued focus on narrow compliance responses can make it hard to capture and analyze field learnings on these issues, especially when elements are outsourced. Access to this knowledge will be highly important to efficiently support both the coming global scale requirements as well as the efficient expansion of strategic outsourcing and virtualization programs across an entire supply network. - Business Value
Companies can engage cross-functional groups across the company to improve knowledge and drive awareness of where new business value may be gained by leveraging product traceability investments. Promising areas of value include enhanced sales and operations planning based on new channel visibility, optimized reimbursement programs from both an efficiency and financial standpoint, and improved brand competitiveness for both established and newly launched products. Taking these benefits into account can help inform initial traceability system design and process reengineering to improve the likelihood of delivering true value for these key business imperatives. - Risk Abatement
A flexible corporate-wide strategy coupled with localizable action plans can reduce risk on several fronts. A baseline strategy improves confidence in the continued access to both markets and channels even as regulations change along with a commensurate reduction in the risk of fines imposed for lack of compliance. Proactive planning can also reduce the risk of corporate or product brand damage due to adverse events by helping identify internal priorities and shaping operational approaches for product traceability investments. Finally, the upfront analysis can identify the often hidden risks arising from resource contention. A comprehensive traceability and compliance solution for dozens or hundreds of product lines will require a wide range of resources, both technical and human. Computing and networking capability, serialization and traceability software, packaging or material handling equipment, and scanning devices are just some of the resources needed. Add to that the human dimension of skilled business analysts, project and program managers, and implementation and integration experts. In-depth planning will uncover the full scope of these resource demands for your company and identify potential availability risks if those same resources will be required by the hundreds of companies in the U.S. and thousands worldwide also deploying traceability solutions for product security and compliance at the same time.
Plan Global, Act Local: One Pharmaceutical Company's Proactive Approach
In reviewing the business and regulatory landscape in light of its diverse product set, one Top 10 pharmaceutical company based in Europe decided that the time to start building an operational playbook was now.
Foundational educational workshops were the starting point. This helped assure that the primary teams assigned to the project understood the current global landscape and were working from the same core set of data. Initial project goals were laid out and the general approach to analysis and planning were defined. The core workshops were extended to selected global teams to both continue education on a broader scale and also facilitate alignment of key managers from the regional organization. An overall global serialization steering committee was also simultaneously defined at this point.
Next, an overall scoping strategy was defined. This helped drive an understanding of the breath of the initiatives, the touch points to various internal systems and impact assessments on operational processes across functional groups.
Feeding this process were a series of internal interviews and discovery meetings which incorporated several technology and process elements that included conversations on data carrier design, primary packaging layout and packaging line system execution, global centralized serialization management architecture and integration approaches to both internal enterprise resource planning (ERP) integration as well as external partners' enterprise systems. The outcome was a set of high-level schedules and rough estimates for cost based on various approaches, refined to the market, product, site and line level, which provided a strong baseline to work from.
Two additional analyses were undertaken. First, benchmarking was performed to evaluate current company position vis-a-vis 20+ company peers as well as the general state of the industry. Secondly, a broad business value analysis was conducted to document and quantify the strategic planning, decision-making and operational efficiency gains to be made from adoption of global traceability and serialization solutions.
The outcome of this project provided several valuable results. The company gained a documented, flexible strategy for meeting current and emerging global serialization initiatives. A prioritized and phased deployment schedule was developed coupled with a baseline cost analysis given different deployment scenarios. Finally, the potential for hard and soft ROI across different business operations was quantified to help feed into the corporate decision-making process for the project.
Conclusion
The myriad of business and regulatory issues confronting most companies along the global pharmaceutical supply chain can be daunting. Against such uncertainty, it is understandable that the temptation is to take the path of least resistance and isolate and tackle each issue as it comes. Unfortunately, it is becoming clearly apparent that the industry has reached a tipping point where this approach will no longer work. The operational costs and business risks are just too high.
Moving forward in developing a "plan global, act local" business strategy isn't easy but it can be done. It is important for companies to understand their unique internal date drivers as they plan their projects. Risk factors exist in all phases of their projects including strategy development, planning, solution design & implementation, and trade partner on-ramping. Those risks span legal interpretation of requirements, business process re-engineering, IT architecture design, technology analysis and section, and proper alignment with supply chain trading partners. It is only with a clear analysis of these issues and the potential benefits of each investment to the whole of the company that a firm can move forward with confidence.
Why Now? The answer is fairly clear. Time is of the essence and in the near-term, development of an effective and logical plan of action is the critical next step on the path to secure compliance and business success. If not now, then companies may want to ask themselves the question: At What Risk?
Brian Daleiden is Director of Marketing for SupplyScape Corp. SupplyScape is a provider of software and consulting services to secure the value and safety of the global pharmaceutical supply chain. http://www.supplyscape.com/
Interested in information related to this topic? Subscribe to our weekly Value-chain eNewsletter.