Operational Savings: How P&G, Unilever Benefit from Shorter Product Hold Times
From global giants P&G and Unilever to niche players like Sage Products, manufacturers have saved millions of dollars with a lean quality approach that eliminates days of product hold time throughout the manufacturing cycle.
These savings result from the use of rapid screening technology on raw materials, WIP and finished goods.
Facilities currently testing finished product for contamination can typically implement this supply chain improvement project within 3 months and free upwards of $400,000 of working capital at a single plant in the first year.
Eliminating Idle Time
A key target of any lean initiative is to identify and eliminate idle time: when materials or people are simply waiting to move on to their next, value-adding step in the production cycle. Process manufacturers that hold goods in inventory or in their distribution system while awaiting the results of quality testing are accumulating multiple days' worth of costly idle time in the production cycle.
"Using a rapid method gives us a distinct advantage,” said Neil Lewis, Global Microbiology Leader, P&G. “We were able to go from holding product for two, three or four days to 24 hours. This has a direct impact on costs and cash availability. It minimizes the inventory we have to hold, and it gives us a responsiveness to our customers.”
About Rapid Screening Technology
Rapid microbial screening for product release is the new industry standard. The traditional agar-plate-based microbial test is a time-consuming method of culturing product samples and waiting to see if microorganisms grow to subjectively visible levels. By contrast, fast, objective and reliable results are achieved 50-80% faster with a rapid screening system.
“Since the technology allows us to get micro results in half the time, we are able to release products and ship products pretty much as needed,” said Mark Entrup, USA Corporate Microbiology, Kao Brands (Cincinnati). “We have reduced our warehouse space significantly. That is, instead of having to produce and then stockpile, the technology allows us to produce as orders require.”
Along with P&G and Colgate, consumer goods giant Unilever adopted rapid methods globally in the mid-1990s “for reducing warehouse, to reduce stock holdings; and to get microbiological results early,” said Peter Jay, UK-based Manager, Hygiene and Personal Care (HPC) Products.
The company has standardized on the use of rapid screening for many of its food and beverage product lines as well. André van Zuijlen of Unilever Nederland describes the technology as “a reliable and efficient method for quickly screening sterilized food products…a state-of-the-art tool.”
Case Study by the Numbers
A mid-sized cosmetics and personal care products manufacturer with $75,000 in average daily value of finished goods was holding final product for 6 days awaiting test results. Raw materials were subject to a 3-day delay while formulated, in-process bulk product was held an average of 4 days prior to packaging.
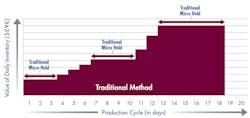
As shown in Figure 1, with a manufacturing cycle of 18 days, over $900,000 in working capital was committed to work-in-process inventory at any point in time.
Fig. 1: Working Capital Investment – traditional methods
Products held in quarantine add no value in lean terms, yet tie up considerable working capital.
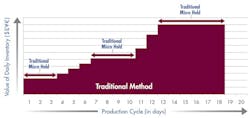
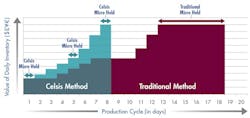
Figure 2 illustrates the difference after the company implemented a rapid product screening system. With microbial test results available in 24 hours, materials move more quickly through the production cycle. By shortening the manufacturing cycle to just 8 days, the company reduced its working capital investment in WIP to approximately $370,000. Implementing rapid screening released $555,000 in working capital.
Fig. 2: Working Capital Investment – rapid method
Faster product release testing shortened the production cycle by 10 days and released significant working capital.
Cost Reductions and Cost Avoidance
After implementing a rapid product screening system, you can re-allocate your new-found floor space to increase manufacturing capacity, add more SKUs to inventory, or reduce distribution center costs. All are paths to improved warehouse utilization.
“We were increasing in volume,” explains Rod Manwill, quality director of Sage Products (Cary, Ill.). “We either had to purchase, install and implement additional production storage tanks and cleaning equipment, or find a way to turnaround our testing in a shorter period of time,” he said. “We avoided the need to bring in expensive equipment, as well as the time, money and effort that would be required to do so.”
Cost avoidance takes on further significance when the rapid method detects a potential contamination issue in 24 hours. Faster detection equals faster correction and recovery, and at a lower cost.
Waiting for quality testing is expensive. When considering an investment in rapid screening, it is critical to weigh the incremental testing cost for the microbiology department against the significant financial benefits that accrue to operations. By eliminating days of idle cycle time, companies adopting rapid screening reduce inventory and safety stock requirements, respond more quickly to customers, recover faster from contamination events and free up working capital. No wonder it is today’s standard for lean manufacturers worldwide.
Jay LeCoque is CEO of Celsis International Ltd., a leading provider of rapid microbial systems for detecting contamination in consumer-bound pharmaceuticals, home and personal care products and beverages.