Creating a Life-Saving Industry – Regenerative Manufacturing
It almost takes a force of nature to move an idea from the lab to the factory.
Luckily for regenerative medicine that force of nature, in the person of Dean Kamen, is on the job.
Kamen, who might be best known as the creator of the Segway or the FIRST Robotics competition, actually has a very long history of creating medical devices.
While still in college, he invented the first wearable infusion pump, which was used in the fields of chemotherapy, neonatology, and endocrinology. In 1976, he founded AutoSyringe, Inc., to manufacture the pumps and at age 30, he sold that company to Baxter International Corp. By then, he had added a number of other infusion devices, including the first wearable insulin pump for diabetics.
Next, he founded DEKA Research & Development Corp. which developed HomeChoice, a peritoneal dialysis system which allowed patients to be dialyzed in their home. And at the request of DARPA Kamen also created Luke, an advanced prosthetic arm in for injured soldiers. He invented the iBOT (electric wheelchair) and most recently created Slingshot a water purification device that is installed across the world.
Kamen talked to IndustryWeek to explain how his vision for bringing regenerative medicine to full-scale production led him to find the necessary resources and leadership that culminated in the creation of Advanced Manufacturing Regenerative Institute/BIOFabUSA.
In creating any industry the vision must come first.
“As a society, we have advanced through the four stages of dealing with disease,” Kamen explained. “The first is to identify the disease and in this stage, we basically watched people die and then figured out what the disease was. The second stage is learning how to treat disease. This is an improvement, but not optimal. The third stage is to become more active, for example when we are able to transplant organs. But it’s the fourth stage where the future lies. It’s where we prevent disease, for example using vaccines. And in the case of regenerative medicine, we build new organs from our own cells. That’s the optimal solution.”
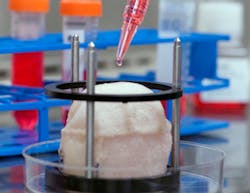
Building organs in the lab is not the stuff of fiction, it’s happening right now. Across the country in many labs, scientists have been working hard to create tissue and pieces of organs, through 3D printing. The advancements, supported by both private and government funding have been gaining quickly. (Note: IndustryWeek has reported on this topic, see Next Wave-Manufacturing Organs, and Expanding Medical Manufacturing.)
The Current State of Industry
One of the first big breakthroughs was in 2006 when Dr. Anthony Atala, director of the Wake Forest Institute for Regenerative Medicine in Winston Salem, N.C. made history by growing and implanting a bladder into a human patient – the first time such a feat had ever been accomplished.
Currently, Dr. Alala uses the patient's own tissue and expands the cells outside of the body creating new tissues and organs that can be put back into the body. The 3D printer creates customized scaffolding that enables the process.
In 2016 researchers at Wake Forest Baptist Medical Center announced that they had printed ear, bone and muscle structures and successfully implanted them into animals. The structures, after being implanted, matured into functional tissue and sprouted new systems of blood vessels, and their strength and size mean that they could feasibly be implanted into humans in the future.
The need for these organs cannot be overstated. Every 30 seconds a patient who could have been saved with tissue replacement dies.
Science has been marching on and at the close of 2016, there were 804 clinical trials underway, with numerous approved and/or marketed products worldwide, and many approved to be marketed in specific regions and countries, according to the Alliance for Regenerative Medicine. The group has a list of products that have been approved by internationally-recognized regulatory agencies, as well as products brought to market in the U.S. under The Food, Drug and Cosmetic Act and The Public Health Service Act.
Moving From the Lab to the Factory
“The research community has done a tremendous job in developing the field of regenerative biology,” says Kamen. There have been significant breakthroughs in cell biology, biofabrication and materials science in the last decades which have laid the foundation for large-scale manufacturing and commercialization of engineered tissues and tissue-related technologies, including tissues- and organs-on-chip.
“Now it’s time to move out of the lab and into the factory and for that you need a different model,” says Kamen.
The current model for industry and pharmaceutical companies, explains Kamen, is to only produce something that can be scaled up quickly with low risk. However, the tissue engineering field is fragmented and lacks a mechanism with which to turn laboratory breakthroughs into manufactured products. And this is where Kamen stepped in, with a team behind him, and created BioFab USA.
BioFabUSA is part of the ManufacturingUSA national structure and under the Advanced Regenerative Manufacturing Institute (ARMI), a non-profit organization located in Manchester, N.H. BioFabUSA is the mechanism through which ARMI will realize its goal. The company brings together the expertise of a variety of fields, both in science and technology to bring the production of organs to scale.
Its mission is to integrate innovative cell and tissue cultures with advances in biofabrication, automation, robotics, and analytical technologies to create disruptive research and development tools and FDA-compliant volume manufacturing processes.
“If we look at the history of manufacturing, we have spent 100 years to get quality up and costs down based on an entire manufacturing ecosystem says Kamen. “We want to do the same and create a roadmap for scaling up biologic output.”
While that’s a tall order, Kamen feels comfortable predicting that within in a few years --two to five - he will bring a product to market.
Why this confidence? His answer is that he has gathered the support of experts in a variety of fields who will bring their experiences together to create this new manufacturing sector. In recruiting his team Kamen started with the company most known for automation and sensors, Rockwell Automation.
Blake Moret, CEO of Rockwell Automation saw the opportunity. “This is literally a life-changing approach and adds a new chapter to medicine,” said Moret in January 2016. “Our contribution is to integrate biomanufacturing science with production techniques that increase the capacity, speed, modularity and consistent quality of new tissue and organ production,” said Moret.
Kamen also sought out Boston Scientific approaching the 80-year-old founder to secure the company’s support. He enlisted Martine Rothblatt, the CEO of United Therapeutics Corp. And Dartmouth University and Worcester Polytechnic Institute joined as did many other companies and educational institutions
One particular win was securing Dr. Richard McFarland and Dr. Becky Robinson-Zeigler, formerly of the FDA, to provide regulatory science expertise. Bringing in regulatory knowledge in the development stage is unusual in the medical device sector. But it certainly fits with Kamen’s drive to bring speed, and urgency to this process.
Once the players were secured Kamen went after funding. He convinced the Department of Defense that his approach, though unusual, was the solution on how to scale up this field. It worked and the Department of Defense provided the initial $80 million.
While the structure of how the funding will flow the organizations is somewhat complicated it essentially follows this format. The DoD funded a new Manufacturing USA Institute (one of twelve such organizations across the country), called the Advanced Tissue Biofabrication Manufacturing USA Institute. Biofabrication is defined as the manufacturing industry segment at the intersection of biology-related research, computer science, materials science and engineering that creates innovations, such as biomaterial and cell processing.
The Institute consists of a consortium of 87 partners from across industry, academia, and government. Kamen’s group, led by ARMI, Inc. led the effort to develop this particular institute and have it located in Manchester, N.H.
DoD’s particular interest was that group would develop next-generation manufacturing techniques for repairing and replacing cells and tissues, which “may one day lead to the ability to manufacture new skin for soldiers scarred from combat or develop organ-preserving technologies to benefit Americans waiting for an organ transplant.”
With core funding from DoD Kamen went on to find other funding sources so when ARMI opened there was $300 million available to fund the projects necessary to scale up production.
From a financial standpoint, the endgame is for the organization to become self-sustaining by attracting paying members; providing contract development services; providing contract manufacturing services and licensing technologies to its members.
The organization, working through a public-private partnership has set out specific goals:
- Develop disruptive cell- and tissue-based technologies across five thrust areas/ The thrust areas: (1) Cell Selection, Culture and Scale-up, (2) Biomaterial Selection and Scale-up; (3) Tissue Process Automation and Monitoring; (4) Tissue Maturing Technologies and (5) Tissue Preservation and Transport
- Produce modular and scalable GMP-compliant manufacturing processes and integrated technologies across technology- (TRL) and manufacturing-readiness levels (MRL) 4-7
- Develop and standardize manufacturing best practices throughout the industry that are aligned with existing and evolving FDA guidance,
close the skills gap in tissue and organ manufacturing by providing training opportunities to undergraduates, graduates, veterans and non-college bound youth.
Workforce for New Manufacturing Sector
Yet another interesting aspect to the creation of regenerative manufacturing is the location. Kamen, who has a strong tie to Manchester, N.H., saw the historic Manchester Millyard as an ideal location to create a new industry. The group of buildings and factories was once the location of the textile capital of the world prior to WWI. Kamen began purchasing property in the area in the ’80s and housed DEKA there. This is where ARMI is now located.
“The creation of this industry in Manchester will bring more high tech companies and talent to the area,” explains Commissioner Taylor Caswell of the Department of Business and Economic Affairs of New Hampshire. “ An entire hub of manufacturing will become centered here. And to achieve that we are looking for companies that want to be suppliers, as well as innovators in this field. As companies come here the talent pipeline will continue to expand on the foundation of manufacturing that has a long tradition in the area,” says Caswell.
The city and state have a number of programs currently underway and are planning others that will connect all educational institutions to provide the skills necessary in this new field. And BioFabUSA has as its goals to close the skills gap in tissue and organ manufacturing by providing training opportunities to undergraduates, graduates, veterans and non-college bound youth, and disseminate knowledge and enabling technologies to encourage continued innovation.
To achieve this goal, BioFabUSA will bring together engineering, life science, computer science, materials science, manufacturing and workforce development expertise from industry, 2- and 4-year community colleges and universities, non-profit organizations, and local, state and federal government. A diverse, multidisciplinary consortium has been established.
Future is Fast Approaching
With all of these pieces in place, Kamen says the realization of regenerative manufacturing is very near.
“We need to keep the focus on staying on the path to high volume,” says Kamen which is why he is reaching out to companies to create a supply chain in the area. He wants companies to look to this new field and find their place in it. Perhaps it takes the form of adapting current products or creating new lines. He wants smaller companies to join this innovative effort, as they can find the resources they need through the research being conducted at BioFabUSA. He wants entrepreneurs to focus their ambitions on this field. He feels this area can be for regenerative medicine what Silicon Valley has been to high-tech.
The ramifications of creating a new industry are far-reaching and occur on many levels. The first level is an entirely new industry whose products will bring new wealth to the country. On another level, it will create a new class of workforce with a higher level of skills and also higher levels of pay. From the perspective of the healthcare industry, on a macroeconomic level, the current expenses associated with treating chronic ailments will be greatly reduced. And you can extrapolate, as Kamen likes to do, where these funds could be redeployed to solve other pressing issues.
And on the personal level, disease will be viewed differently. “How would the entire system of healthcare change, and someone’s personal health diagnosis change, if we could print a new kidney or liver when you needed one,” Kamen said.
“This will become a reality and in the not so distant future we will look back on our current medical system in disbelief at our limitations.”